The rise of virtual clinical trials and advancements in technology has been seen to occur in tandem highlighting the importance of the inclusion of technology in the conduct of trials. Virtual clinical trials help bridge the divide between trials and clinical practice by helping overcome challenges associated with traditional site-based trials such as the inconveniences in travel to central sites, low rates of patient recruitment and retention, reduced patient diversity, and financial burden. These reasons are contributing to the rapid emergence of virtual clinical trials in the clinical landscape today. These trials are also known as remote or decentralized clinical trials and as the name implies involves the collection of data, patient monitoring, and study visits by leveraging the potential of digital technologies, internet access, and improved affordability and accessibility. In addition to patient-related factors (recruitment, training, safety monitoring), virtual clinical trials are also attractive to pharmaceutical companies/sponsors as they help cut costs by avoiding the maintenance of central sites and staff and decrease time to market of a new drug or treatment by speeding up patient enrolment and decreasing the dropout rates.
Understanding all the benefits of virtual clinical trials is important and it is straightforward to see that the maximum benefits that can be derived from these trials’ hinges upon the use of appropriate technology that is efficient, validated, accessible, and user-friendly. Advances in technology, digitization, and widespread internet access have all facilitated the incorporation of various digital health technologies for the conduct of virtual clinical trials. The various tools that are used in virtual trials are shown in the figure below.
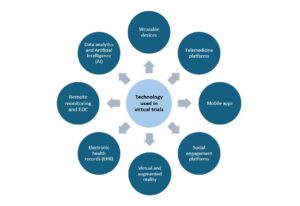
Virtual Clinical Trials Tools-
- Wearable devices: These include smartwatches and fitness trackers that gather information such as heart rate, calories, step counts, heart rate, and sleep patterns that help to monitor a patient’s health status without the need for site visits. This data can be transmitted directly to healthcare professionals (HCPs) and clinical trial staff allowing for real-time monitoring of various physiological parameters enabling action to be taken in a timely manner. Biosensors such as skin patches and internal sensors such as smart pills can also be used that allow information to be streamed directly to HCPs allowing for continuous monitoring of the patient and safety monitoring.
- Telemedicine platforms: Telemedicine eliminates the need for patients to visit central sites and study visits thereby improving patient convenience by providing virtual consultation platforms for patients. This is extremely important for patients in remote areas and provides a means for medical monitoring, patient advice and education, and reminders through phone calls or video appointments.
- Mobile apps: Increased accessibility to wireless and broadband services has greatly increased the percentage of patients that have smartphones allowing them to download healthcare-related apps. Patient-reported outcomes (PRO), electronic diaries, medication adherence details, reminders, patient recruitment, and engagement details can all be captured and greatly improved using various applications.
- Social engagement platforms: These include online communities, support groups, forums, and video chat tools that can be used across all stages of the trial from patient recruitment, follow-up, and remote consultations allowing for engagement between the patients and HCPs. These tools allow for communication and provide medical and emotional support to patients for whom it is not feasible to travel to trial sites due to logistic reasons and physical disabilities.
- Virtual reality (VR) and augmented reality: These technologies simulate the clinical trial environment and can help with training and educational awareness, rehabilitation, and conduction social and behavioural data studies. Motor rehabilitation, pain management, and cognitive rehabilitation can be conducted by VR simulations and games. Educational content and training can also be delivered through VR platforms allowing for better patient engagement and awareness and study data can be captured in a standardized manner.
- Electronic health records (EHRs): Digital capture and storage of patients’ medical information in electronic records can enable sharing between investigators at different sites and help with patient recruitment programs. EHRs allow for collection of data in a standardized, objective way that helps in data sharing and analysis especially in multi-site studies.
- Remote monitoring devices and electronic data capture (EDC) systems: remote monitoring devices enable patients to perform measurement of various physiological parameters at home and transmit this data to trial staff to analyze it. EDC systems enable data capture from remote devices in real-time using electronic case report forms (eCRFs) and creation and submission of data reports to regulatory agencies.
- Data analytics and artificial intelligence (AI): These methods help in analyzing large volumes of data from virtual trials that help in analyzing trends and outcomes as well as refining trial protocols as the study progresses. AI helps in several aspects of trial protocol design and development by using past data to inform improvements in the protocol, help in patient recruitment by matching patient characteristics to trial eligibility criteria, real-time safety signal and adverse event monitoring, and more recently allowing for outcome prediction based on the concept of digital twin or virtual replicas of patients.
Therefore, the above technologies when used appropriately in trials have the potential to be disruptive and change the way trials are conducted. Remote monitoring technologies although popular initially have become more-popular ever since the COVID-19 pandemic and as technology progresses are here to stay. It is important for sponsors, investigators, HCPs, and regulatory agencies to be knowledgeable about the use of these technologies as they provide not only a more efficient and cost-friendly approach but are also patient-centric.
References
- How artificial intelligence could redefine clinical trials in cardiovascular medicine: lessons learned from oncology
- The Evolving Role of Decentralized Clinical Trials and Digital Health Technologies | FDA
- Decentralised clinical trials: ethical opportunities and challenges – The Lancet Digital Health
- Transforming Clinical Trials with Technology – Neuroscience Trials of the Future – NCBI Bookshelf (nih.gov)

