Digital technology and the COVID-19 pandemic have catapulted the rise of virtual, decentralized, or Hybrid Clinical Trials. Unlike traditional trials in which almost all the trial activities such as patient recruitment, consent, and site visits involve face-to-face contact between the participant and healthcare provider or clinical trial site staff, virtual or decentralized trials make use of remote technologies and digital platforms for all or some of the trial processes. The virtual trial clinical market is estimated to reach $13.78 bn by 2027, registering a CAGR of 12.6% from 2020-27 (1). The term virtual, ‘site-less’, remote, or fully decentralized generally refers to trials in which all trial activities from patient recruitment to data analysis are conducted remotely. Whereas the term ‘decentralized’ or ‘hybrid’ implies that some of the activities require the participants to visit the sites whereas some activities can be done using digital platforms. The Clinical Trials Transformative Initiative (CTTI) defines decentralized clinical trials (DCT) as studies executed through telemedicine and mobile/local healthcare providers (HCPs), using processes and technologies differing from the traditional clinical trial model (2).
 The decentralized approach involves the use of digital tools such as telemedicine or electronic communications/platforms to bring hybrid clinical trials processes such as consent and assessments closer to patients. The benefits of hybrid Clinical trials are myriad:
The decentralized approach involves the use of digital tools such as telemedicine or electronic communications/platforms to bring hybrid clinical trials processes such as consent and assessments closer to patients. The benefits of hybrid Clinical trials are myriad:
- Reduced patient burden by eliminating or decreasing the number of on-site visits which is particularly beneficial for elderly or immobile patient populations and those that live far away from trial sites
- Increased and faster patient recruitment as they allow for patient participation from places that would otherwise be difficult to penetrate based on a lack of healthcare infrastructure
- Improved patient adherence and engagement as the patients can self-administer medications in the comfort of their own homes
- Increased patient diversity as patients can be recruited from different geographical locations with different ethnicities reflecting the real-world scenario
- Decreased or eliminated costs associated with site staff management and site maintenance
- Better accessibility for patients to novel treatments that would be otherwise difficult to obtain especially in cases of rare diseases
- Collection of patient data in real-time that allows for continuous measurements of vital signs and other parameters allowing for interim analysis, alterations in allocation ratios to favor the effective treatments, design of optimal dosage regimens, and early stopping of trials based on efficacy data
- Rising comfort and accessibility along with reduced costs associated with the use of digital technology such as sensors and wearable devices by patients
However, despite the obvious advantages associated with virtual approaches in hybrid clinical trials some challenges still exist. These involve regulatory challenges, hesitation, and in part lack of knowledge and training on part of healthcare professionals to use digital platforms, inability to incorporate remote patient monitoring for all types of studies limiting their applicability, and data security concerns. Hybrid Clinical Trials in some way help to overcome these limitations by incorporating the best of virtual and traditional clinical trial approaches and leveraging the use of virtual approaches when needed in parts of the hybrid clinical trials in which they are expected to be most beneficial. Furthermore, regulatory bodies such as the United States Food and Drug Administration (FDA) have released guidance on digital health technology (DHT) for remote data collection highlighting the openness of regulatory bodies to the use of virtual approaches.
Trends in Hybrid Clinical Trials
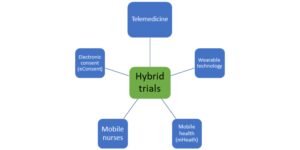
Considering the prime importance of safety and efficacy of novel treatments, it is essential that all types of clinical trials, whether traditional or hybrid keep patient safety, well-being, and confidentiality at the forefront of all their operations. Various virtual tools have emerged in the conduct of decentralized or Hybrid Clinical Trials that are also ever evolving with digitization. The following figure depicts the recent trends in hybrid clinical trials that allow for remote monitoring:
- Telemedicine
Telemedicine involves the use of technology such as video conferences or messaging systems to facilitate communication between physicians and patients allowing for treatment or diagnosis of diseases. Benefits of telemedicine for the patient include elimination of travel time, suitability for immobile or elderly patients, accessibility to specialist physicians, and protection from the contagious environment of clinics and hospitals. Telemedicine for clinical trials platforms also allow for more efficient follow-up of patients, especially in the case of chronic conditions. The adoption of telemedicine gained traction during the COVID-19 pandemic by bridging the gap between participants and healthcare professionals and streamlined patient monitoring and recruitment enabling trials to progress without any interruptions caused by travel restrictions and safety concerns. The continued use of telemedicine requires that the platforms used be simple for patients and site staff and that the technology is secure and patient data remains confidential. However, it is important to note that not all types of Patient behavior monitoring application are suitable to allow for virtual consults and may require in-person site visits.
- Wearable technology
Wearable devices include fitness trackers, blood pressure and ECG monitors, smart health watches, and biosensors. These devices include sensors for the measurement of biometrics and a system for continuous communication and sharing of data with healthcare professionals enabling the real-time collection of patients’ vital signs and other measures that can guide drug dosage and treatment. Abnormal data can signal safety issues that can be treated before an adverse event occurs. Furthermore, the information from wearable devices increases patient awareness of their health and thus engagement in the trial process. The incorporation of wearable devices in trials requires them to be accurate such that the information that is obtained from them and that is used to make critical decisions is reliable. Additionally, proper positioning of wearable devices and biosensors is also critical and may require patient training.
- Mobile health (mHealth)
The ubiquity of the internet and mobile phones has prompted the rise in the use of mobile devices, smartphones, tablets, monitoring devices, and personal digital assistants for medical and public health purposes (3). This technology enables physician-patient communication and allows the delivery of important messages via SMS regarding dose schedules and protocol-related information. Advanced features of smartphones such as GPS tracking and collection of signs such as pulse, oxygen saturation, and activity tracking allow for continuous tracking of the patient and wireless transmission of this information to the site. mHealth enhances patient recruitment and retention by providing access to specific studies, social media engagement, messaging reminders, and training programs built for the patient. Despite these obvious advantages, it is important to maintain data privacy and ensure that the features are compliant with regulatory agencies.
- Mobile research nurses
Successful implementation of the hybrid design for hybrid clinical trials should also consider the often-forgotten human-centric component in addition to the digital element. This is especially important for elderly or pediatric populations that may not have access to or be familiar with the operation of wearable and mobile devices making face-to-face interaction with health care professionals an important part of their trial experience. Mobile research nurses are trained in aspects of good clinical practice (GCP) conduct and adverse event (AE) and protocol deviation reporting and are proficient in collecting samples and performing tests in patients’ homes. Physical visits with mobile nurses are expected to increase patient confidence and comfort and provide personalized attention when needed.
- Electronic consent (eConsent)
Electronic Informed consent from participants in hybrid clinical trials is one of the most crucial aspects and is connected strongly with ensuring patient well-being and safety. It is often difficult for participants to fully understand the technical and scientific aspects of a hybrid clinical trials which can increase hesitancy in trial participation. Furthermore, some participants may feel pressurized to make decisions in the presence of trial staff making it important to focus on developing methods to increase patient comfort and make them aware of any benefits and risks of the trial. Remote consent or electronic consent forms can be designed to allow participants to get details on any points that require further explanation and allows the recruitment of a much larger and more diverse population. Collection and storage of forms is also more efficient using digital platforms compared to paper-based forms at each trial site.
Therefore, although hybrid clinical trials are touted as the trial design for the future, their implementation requires the concerted effort of sponsors, participants, and regulatory agencies to maximize their potential and make them the mainstream approach for the conduct of clinical trials globally.
References
- Polaris Market Research
- CTTI Recommendations: Decentralized Clinical Trials September 2018
- mHealth (who.int)

