Current Challenges in Clinical Trial Patient Recruitment and Enrollment, including Patient Engagement to avoid dropouts.
Introduction: Understanding the Basics of Clinical Trial Patient Recruitment and Enrollment
Clinical trial patient recruitment and enrollment are crucial components of the drug development process. It is a process of identifying, screening, and enrolling participants in a clinical trial to evaluate the safety and efficacy of a new drug or medical intervention. The success of a clinical trial depends on the recruitment and retention of an appropriate number of eligible and willing participants. Here are some basic aspects of clinical trials patient recruitment and enrollment:
-
Eligibility criteria for Patient Recruitment and enrollment:
Each clinical trial has specific eligibility criteria that must be met by potential participants. These criteria are designed to ensure that the trial results are accurate and applicable to the intended patient population. Eligibility criteria may include age, gender, medical history, current health status, and specific disease symptoms or conditions.
-
Recruitment strategies for Patient Recruitment and enrollment:
There are various recruitment strategies (approved by ethics committee) that can be used to reach potential participants, such as advertising through social media, patient advocacy groups, and healthcare providers. In some cases, targeted recruitment efforts may be necessary to reach underrepresented populations.
-
Informed consentfor Patient Recruitment and enrollment:
Before enrolling in a clinical trial, participants must provide informed consent. This means they must receive detailed information about the trial, including its purpose, risks, and benefits, and have the opportunity to ask questions and discuss any concerns with the research team.
-
Randomization for Patient Recruitment and enrollment:
In some clinical trials, participants are randomly assigned to a treatment group or a control group. Randomization helps to ensure that the results of the trial are not biassed and that any differences between the treatment groups are due to the intervention being tested.
-
Retention for Patient Recruitment and enrollment:
Retaining participants in a clinical trial can be challenging, but it is important to ensure the trial’s success. Strategies such as regular communication, providing incentives, and addressing any concerns or issues that arise can help to keep participants engaged and motivated to stay in the trial.
The Current Challenges in Clinical Trial Patient Recruitment & Enrollment Today
Current challenges in clinical trial patient recruitment and enrollment have always been challenging, but today there are several unique challenges that researchers and clinicians must contend with. Here are some of the top challenges:
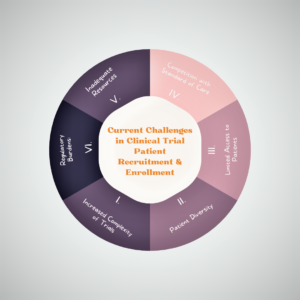
Increased Complexity of Clinical Trial Patient Recruitment and Enrollment:
Modern clinical trials are becoming increasingly complex, with more rigorous eligibility criteria and more extensive protocols. This complexity can make it more challenging to identify and enroll eligible patients.
Patient Diversity of Patient Recruitment and Enrollment:
There is a growing awareness of the importance of diversity in clinical trials, but recruiting and enrolling diverse patient populations can be challenging. Language barriers, cultural differences, and mistrust of the medical system can all be barriers to recruitment.
Limited Access to Patient Recruitment and Enrollment:
In some therapeutic areas, there may be a limited number of patients available for clinical trials, which can make recruitment and enrollment challenging.
Competition with Standard of Care for Current Challenges in Clinical Trial Patient Recruitment and Enrollment :
In some cases, patients may be hesitant to participate in a clinical trial because they are satisfied with their current standard of care.
Inadequate Resources in clinical trial patient recruitment and enrollment:
Challenges in patient recruitment for clinical trials can be resource-intensive, and some researchers may not have the necessary resources to effectively recruit and enroll patients.
Regulatory Burdens for clinical trial recruitment and enrollment:
There are many regulatory requirements that must be met for clinical trial recruitment and enrollment, which can be time-consuming and costly.
A Deeper Look at Solutions for the Common Challenges of Clinical Trial Patient Recruitment and Enrollment
Patient recruitment and Enrollment is a crucial aspect of the drug development process. However, it is also a challenging process that is affected by various factors, such as patient population, trial design, study location, and regulatory requirements. Clinical Research Organization can offer valuable solutions to the common challenges of patient recruitment and enrollment in clinical trials, helping sponsors to achieve their study objectives and bring new treatments to market more efficiently. In below points, we will take a deeper look at some of the common challenges of clinical trial patient recruitment and enrollment and discuss potential solutions.
Lack of Patient Awareness:
One of the biggest challenges in patient recruitment is the lack of patient awareness about the trials. Many patients are not aware of the availability of clinical trials or the potential benefits of participating in them. This can be addressed by increasing patient education and outreach efforts.
Potential Solutions:
- Engage with patient advocacy groups to spread awareness about clinical trials.
- Use social media platforms and online forums to reach a broader audience.
- Partner with healthcare providers and clinics to educate patients about clinical trials.
Eligibility Criteria:
Eligibility criteria are the specific requirements that patients must meet to participate in a clinical trial. These criteria can be very strict, making it challenging to find eligible patients.
Potential Solutions:
- Consider broadening eligibility criteria to include a more diverse patient population.
- Use adaptive trial designs that allow for flexibility in eligibility criteria.
- Use electronic health records and other data sources to identify eligible patients more efficiently.
Location:
The location of the clinical trial can also affect patient recruitment and enrollment. Trials conducted in remote or hard-to-reach areas may have difficulty finding eligible patients.
Potential Solutions:
- Partner with local healthcare providers and clinics to increase patient access to trials.
- Use telemedicine and other remote monitoring technologies to reach patients in remote areas.
- Consider conducting trials in multiple locations to increase patient diversity.
Patient Retention:
Patient retention is critical to the success of a clinical trial. High patient drop-out rates can affect the trial’s statistical power and delay the study’s completion.
Potential Solutions:
- Build strong relationships with patients by providing regular communication and support.
- Implement patient engagement strategies, such as patient portals and mobile applications, to keep patients informed and engaged.
- Offer incentives for patient participation and retention, such as travel reimbursement or gift cards.
Regulatory Requirements:
Patient recruitment strategies in clinical trials are also subject to regulatory requirements that can be time-consuming and complex.
Potential Solutions:
- Use electronic data capture systems to streamline data collection and management.
- Hire experienced clinical research coordinators to manage regulatory compliance.
- Engage with regulatory authorities early in the trial planning process to ensure compliance with all requirements..
How to Leverage Technology for Effective & Efficient Clinical Trial Patient Engagement
There are several ways to leverage technology for effective and efficient clinical trial patient engagement. Here are some of the most effective strategies:
Mobile Applications:
Develop mobile apps that can help patients track their medication, appointments, and other important information related to the clinical trial. These apps can also be used to provide patients with personalized reminders and notifications, as well as access to educational resources. By using mobile applications for patient engagement in siteless clinical trials, we can overcome some of the traditional barriers to participation such as distance, transportation, and time constraints.
Wearable Devices:
Use wearable devices to monitor patients’ vital signs and collect data on their health status. This data can be used to provide personalized recommendations and support to patients, as well as to track their progress in the clinical trial.
Telehealth:
Utilize telehealth technologies to provide remote consultations and support to patients. This can include virtual visits with healthcare professionals, as well as remote monitoring of patients’ health status.
Social Media:
Use social media platforms to engage patients and provide them with up-to-date information about the clinical trial. This can include posting regular updates, answering questions from patients, and sharing patient stories and experiences.
Electronic Health Records:
Utilize electronic health records (EHRs) to streamline patient data collection and management. This can help to improve data accuracy, reduce administrative burdens, and enhance patient safety.
Patient Portals:
Provide patients with access to online patient portals that allow them to view their medical records, communicate with healthcare professionals, and access educational resources related to the clinical trial.
Overall, leveraging technology can help to improve patient engagement and participation in clinical trials, ultimately leading to more accurate data and better treatment outcomes.

